Purification
Using our robust downstream purification platforms, Bioworkshops can develop an efficient downstream process for most mAbs and many other therapeutic proteins in weeks. We offer the flexibility to transfer, develop and optimize client-specific processes for either monoclonal antibodies and recombinant proteins, and can customize processes to meet clients’ special requests. Our goal for downstream process development is to deliver a robust, scalable and cost-effective purification process through yield optimization and product purity improvement.
Case Study
Due to the structure and impurities of bispecific antibodies are more complicated, the experience of downstream purification technicians and the company's platform technology are more critical. A full understanding of the structural characteristics of BsAbs and its major impurities can help to develop effective purification process. Our unique platform process and technicians with extensive experience in the development of BsAbs help our clients in the whole process of biopharmaceutical development. Our goal is to deliver a robust, scalable and cost-effective purification process for our clients by optimizing yields and improving product quality.
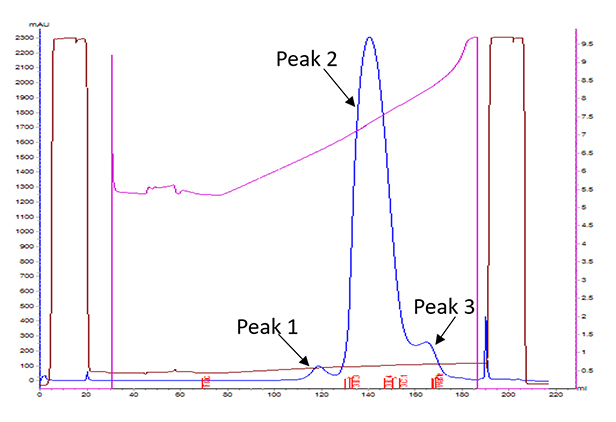
Background:
The case demonstrates a process to improve the purity of BsAbs by mixed-mode chromatography.
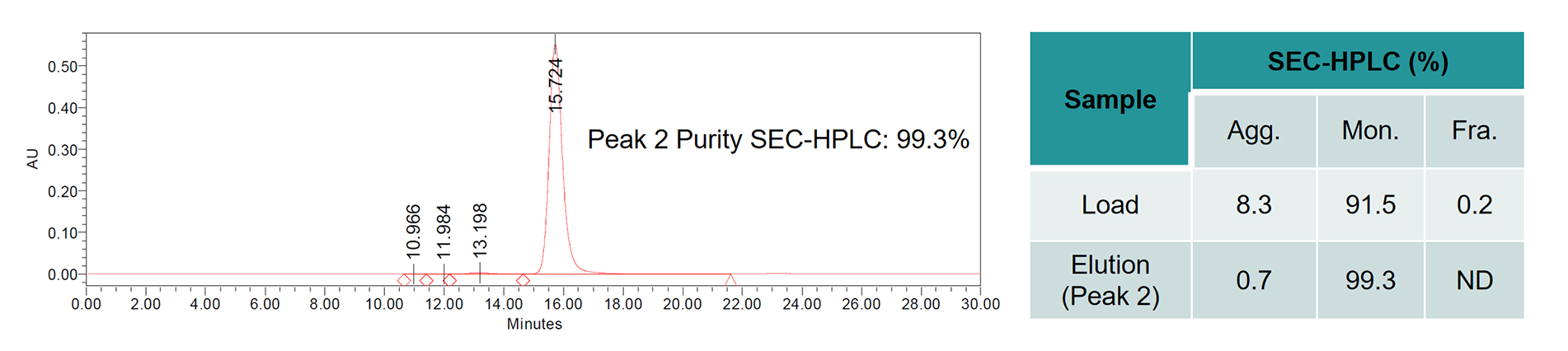
The purity of the sample before purification was 91.5%. Based on the extensive experience of the purification process development team and the unique platform process of BWKS, the purity of the sample was improved to 99.3% (Detection by SEC-HPLC) after process optimization.
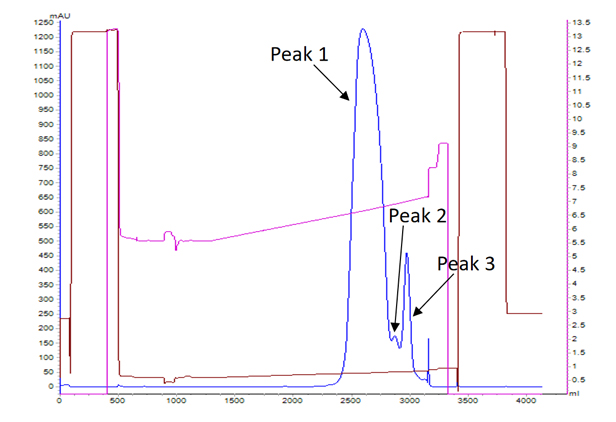
Background:
The case demonstrates a process to improve the purity of BsAbs by CEX.
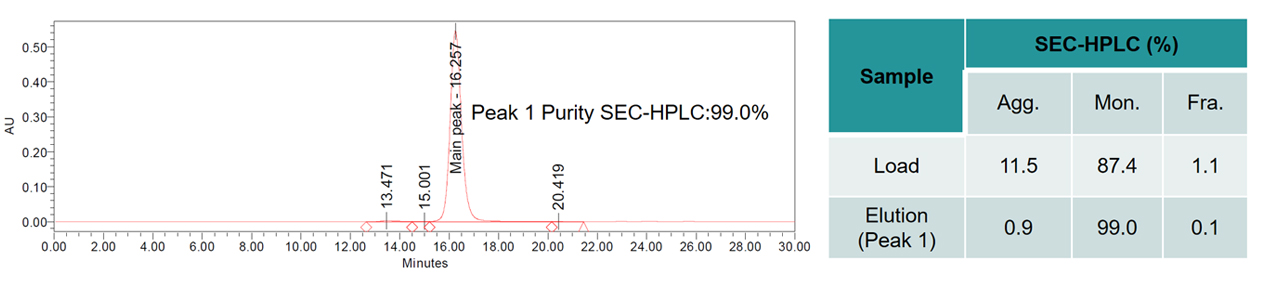
The purity of the sample before purification was 87.4%. Based on the extensive experience of the purification process development team and the unique platform process of BWKS, the purity of the sample was improved to 99.0% (Detection by SEC-HPLC) after process optimization.