Cell Line Development
To obtain the best results for a wide range of cell culture products, Bioworkshops is agnostic when it comes to expression system. We cooperate with many leading cell line development suppliers as well as offering multiple CHO-based expression platforms of our own. Our cell culture leaders will evaluate key indicators of the product to recommend the best cell line construction plan and screening process. Starting from target molecule, DNA or antibody sequence we provide all the services from the vector construction to the screening of stable and high-expressing cell lines that meet the required protein quality for reliable commercial production.
CLD case study
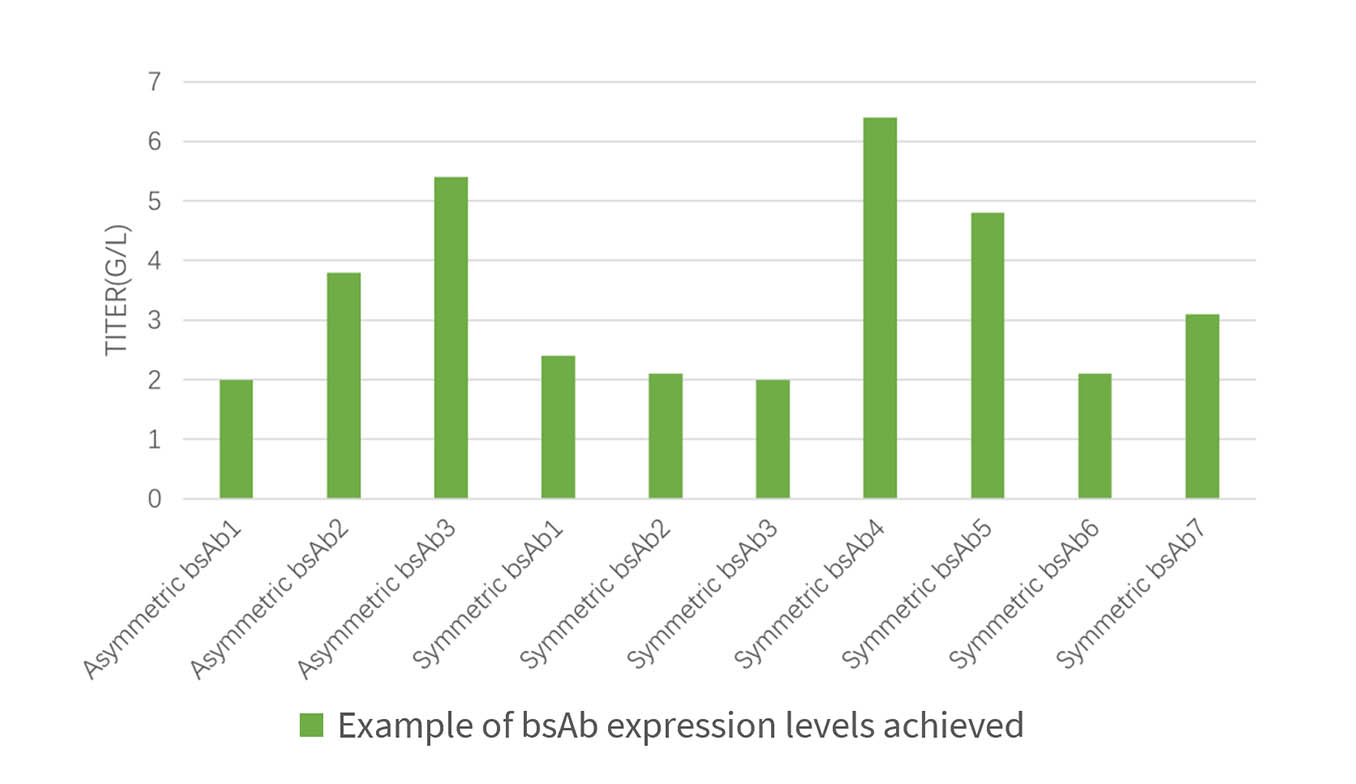
Cell line development is the first step in chemical manufacturing and control (CMC) stage and the basis of process development, which determines quality of final products. Compared with those of monoclonal antibodies (mAbs), Cell line development workload of bispecific antibodies (bsAbs) is larger while development period is longer. How to get a balance between expression level of cell lines, product quality and development time is a challenge that must be faced in developments of bsAbs cell lines. Bioworkshops delivered cell line development projects including mAbs, bsAbs (symmetric + asymmetric), fusion protein and so on with robust process, high expression, scalability and good cell line stability. These projects entered subsequent CMC development stage and clinical research applications that have been successfully approved in the United States, Australia and China. In addition, Bioworkshops can get involved in discovery stage of the partner as well, using professional CMC platform to help screen candidate molecules, shorten the development period, reduce risks in the CMC stage of projects, and push molecules into clinical practice rapidly with robust technology and efficient production.
• Optimized processes for mAb and bsAb cell line development
• 300-1,200 candidates screened in mini-pool phase
• 150-600 candidates in single clone screening
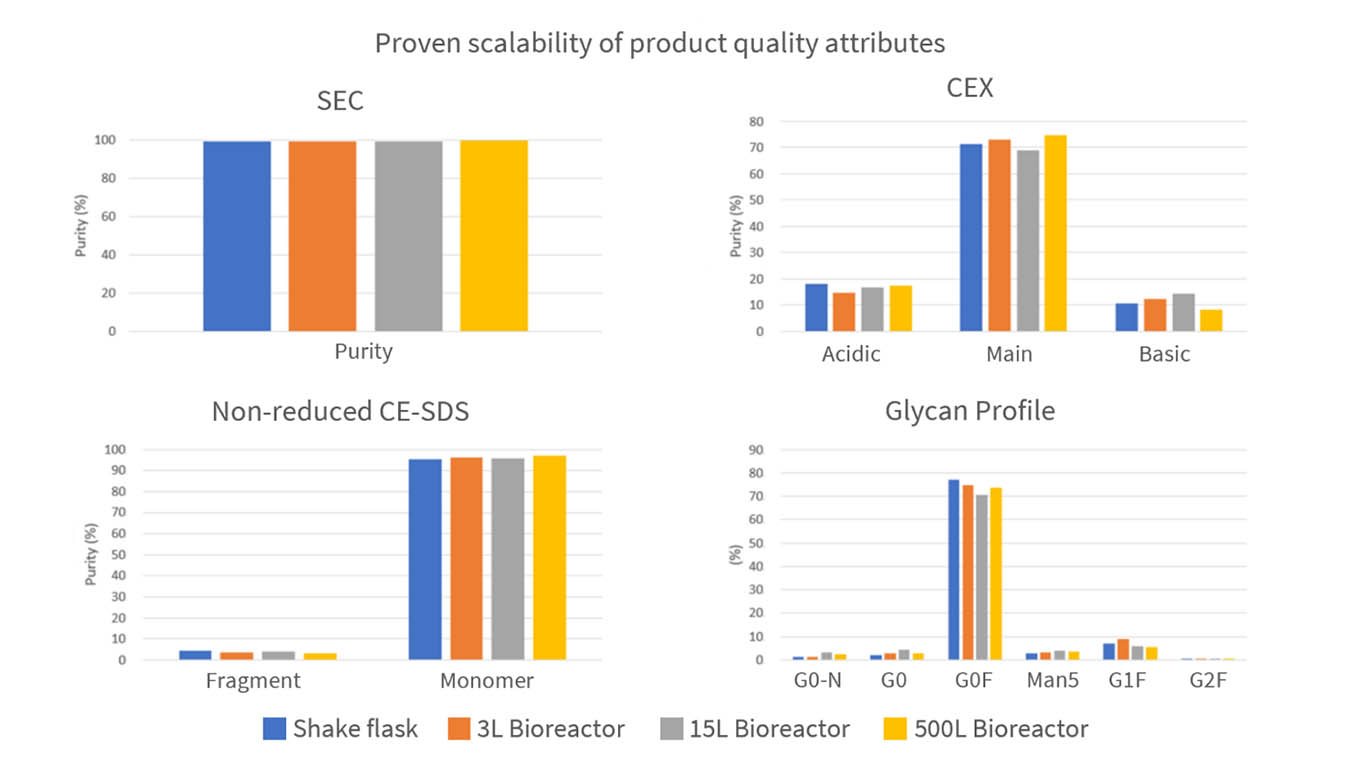
• Guarantee product quality with better expression
• Lower product-related impurities
• Two host cell systems with clear traceability: ATCC CHO-K11 and Merk CHOZN-K1
• Cell line developed following QbD approach
• GMP cell banking service
• Delivery of best clones can be within 4 months of receiving target sequency
1ATCC® CCL-61™ (CHO-K1): The ATCC trademark and trade name and any and all ATCC catalogue numbers are trademarks of the American Type Culture Collection
• CHO expression systems with proven scalability
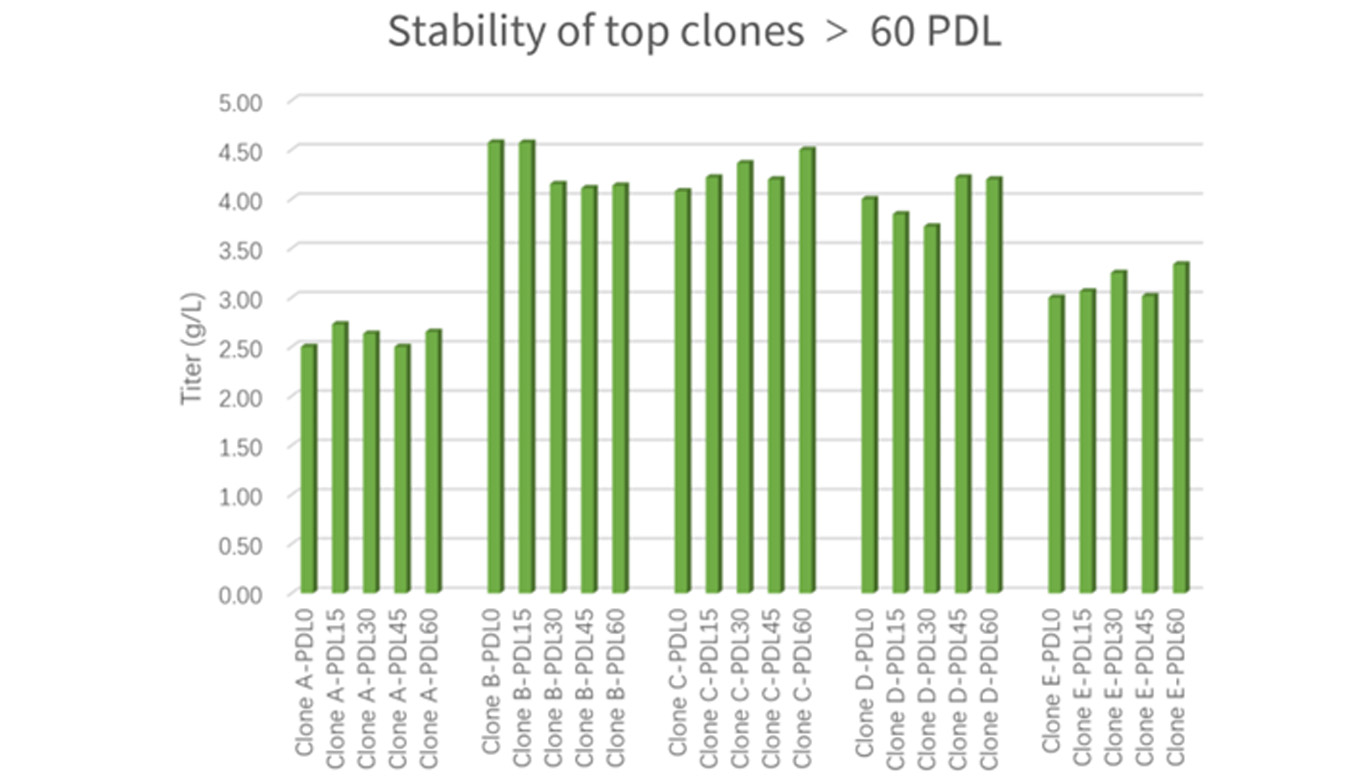
• At least 2 top clones guaranteed to exceed 60 PDL for gene copy number, expression level and product quality
Cell Line development process
~3 weeks
Vector construction
Select suitable vector
Codon optimization
Gene synthesis
Plasmid construction
Target sequence sequencing
~6 weeks
Cell transfection and cell pool/ Minipool screening
Select suitable transfection conditions
Monitor growth and expression 48h after transfection
Cell pool/ Minipool preparation
Top pool/ fed-batch (FB) cell performance assay
Top pool/ Minipool quality analysis
7~8 weeks
Single clone screening
Top pool/ Minipool single clone preparation
Clone images
Top single clone Fed batch cell performance assay
Single clone quality analysis
13~14weeks
RCB banking and stability study
Top 3 RCB Cell Banking (30 vials)
RCB mycoplasma test
60 PDL stability study
FB evaluation every 15~20 PDL
Quality inspection of samples at the start and end points
Quality inspection of samples at the start and end points (Copy number and target gene sequencing)