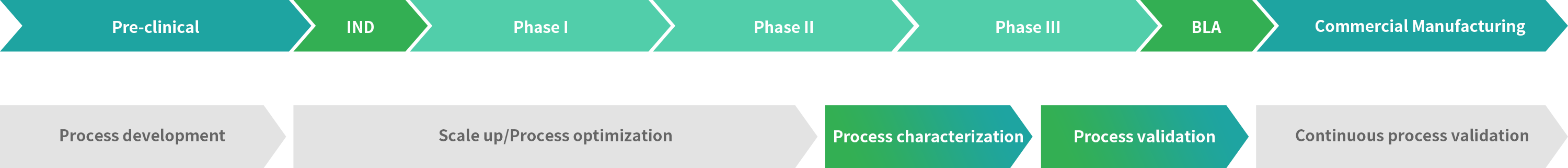
PC、PV
Process Characterization
A set of documented studies in which operational parameter are purposefully varied to determine their effect on product quality and process performance.
Process Validation
The collection and evaluation of data from the process design stage to commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality products.



 Experienced
Experienced One-stop Solution
One-stop Solution Full platform
Full platform Quality assurance
Quality assurance Compliance
Compliance



