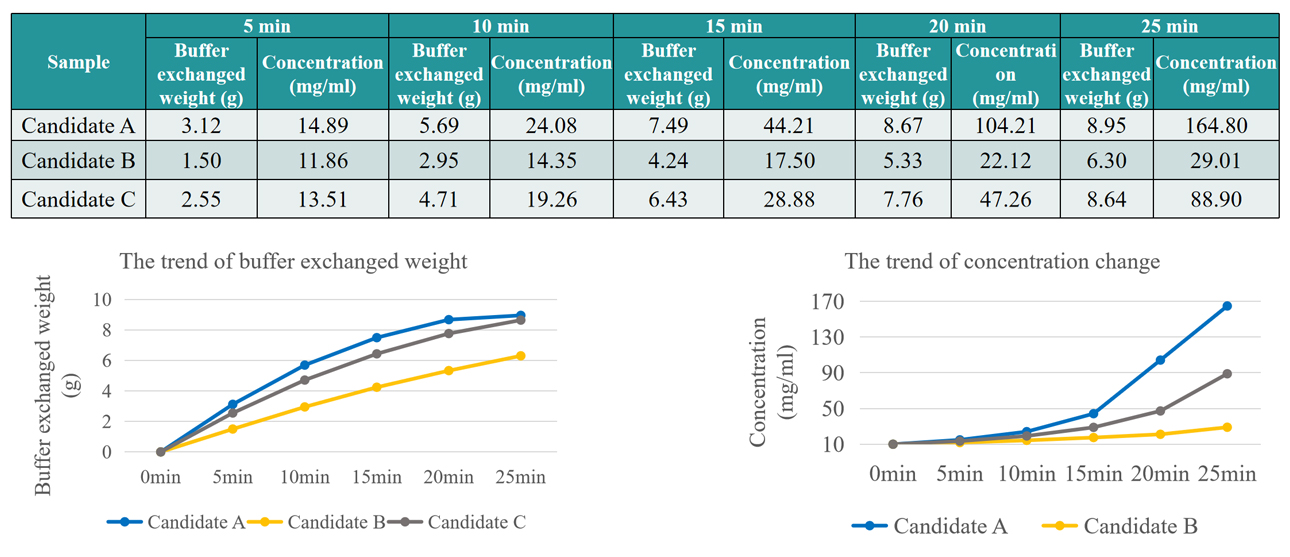
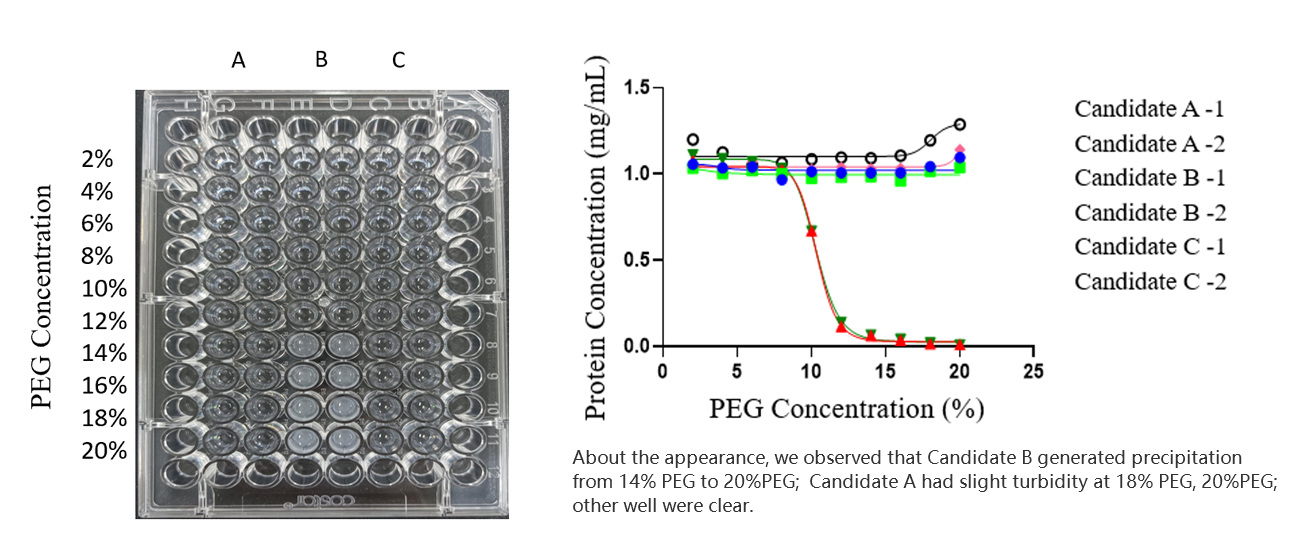
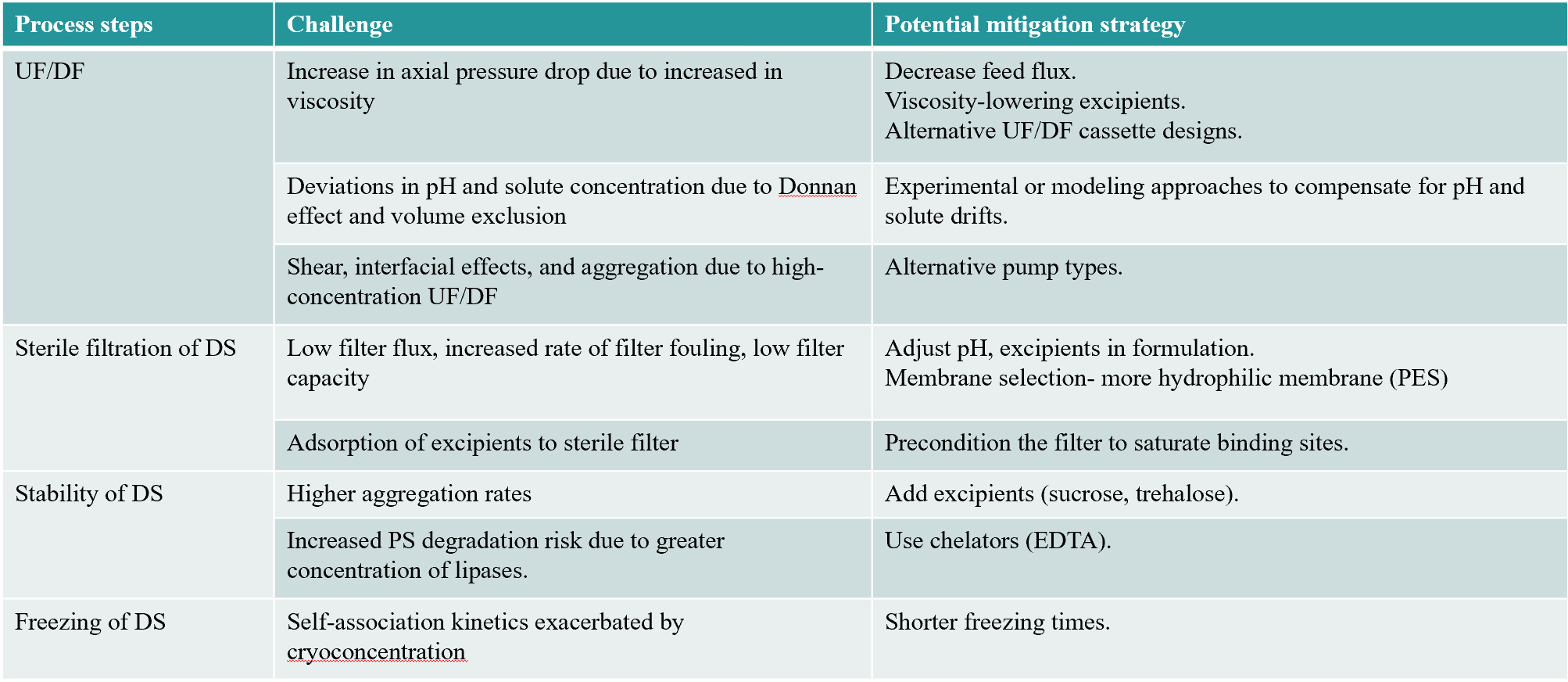
Formulation
Our Formulation Development team offers services to develop customized formulations for different biologics, such as monoclonal antibodies, bi-specific antibodies, single-chain antibodies, nano antibodies, recombinant proteins, and fusion proteins . Key capabilities include filling process and formulation characterization (DoE), molecule developability and high concentration formulations.