DNA to IND and BLA. One-Stop Solution

Bioworkshops is a full-service biologics CDMO focused on antibody products. We are committed to offering cost-effective, high-quality, and efficient outsourcing solutions that assist our clients shorten the time and reduce the cost to start clinical trials and enter USA, EU, and APAC markets.
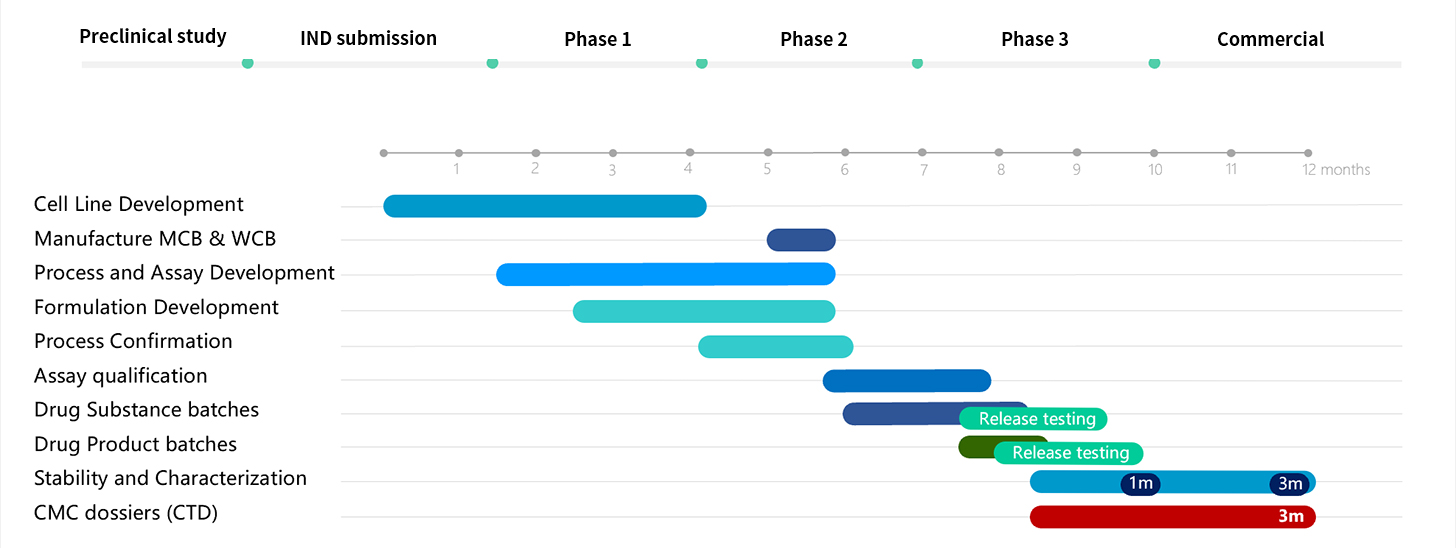
Our scope of services covers product development from gene sequence to finished product including cell line development, process development, analytical method development, formulation development, clinical and commercial drug substance manufacturing (200L to 2000L), finished product manufacturing (vials, lyophilized product, prefilled syringes, and cartridges for 1,000 to 100,000 units), process characterization, process validation, full product testing, and preparation of CMC submission dossiers. We assure our clients' products quickly obtain approval in the highly regulated markets of USA, EU, Australia, and China as well as other regions.


Bioworkshops holds an NMPA Drug Manufacturing License and successfully passed EU QP and US-FDA GMP compliance audits for development and manufacture of drug substances and drug products.

We assure our clients' products quickly obtain approval in the highly regulated markets of USA, EU, Australia, and China as well as other regions.

Within one year we manufacture more than 120 GMP batches covering 200L, 500L, and 2000L of drug substance and drug product.
Great production flexibility with single-use platforms from Sartorius, Cytiva, and Thermo at scale up to 2,000L plus high-capacity fill and finish lines that cover all dosage forms commonly used for biologics, including vials, lyophilized product, prefilled syringes, and cartridges.

With strong quality systems, as well as in-depth experience of ICH, EMA, FDA and NMPA regulations, we provide clients the best solution and services for their biologic products.