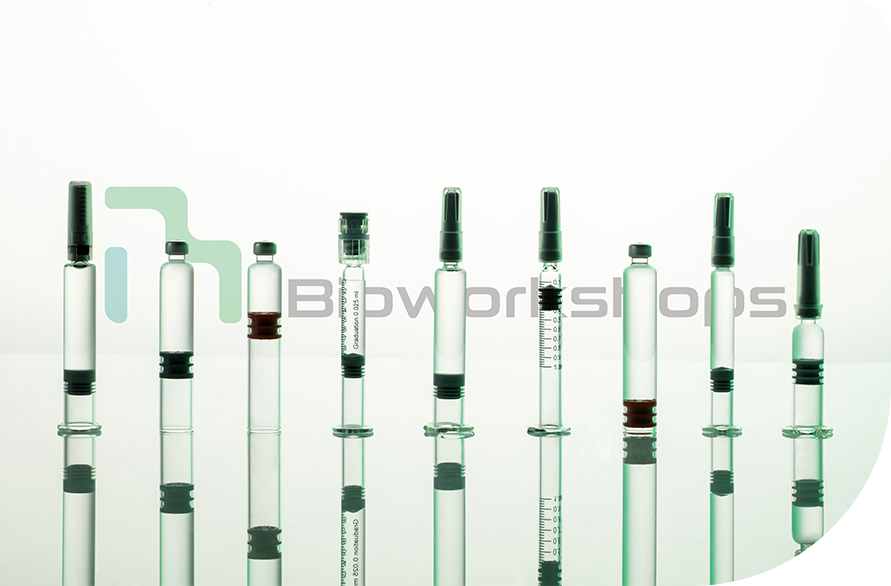
PFS、Cartridges
Bioworkshops seek to provide a complete one-stop service for our clients to global standards. Aseptic fill & finish capacity covers clinical & commercial services for vials, prefilled syringes (PFS), and cartridges in compliance with US and EU GMP with annual filling capacity of 20 million units.
With fully automated systems, Bioworkshops lines operate with minimal over-fill volume and improve the utilization rate to effectively increase capacity and reduce unit cost with minimum risk of contamination from operator intervention.
With rich experience in aseptic manufacturing, meticulous production processes using high-performance equipment, Bioworkshops provide a more efficient fill and finish service to our clients.

-

Filling volume range: 0.2mL-5.0mL
-

Maximum lot size: 100,000 syringes
-
Line speed up to 12,000 units/h
-

Robotic arm in aseptic processing area (APA) to minimise risk of contamination