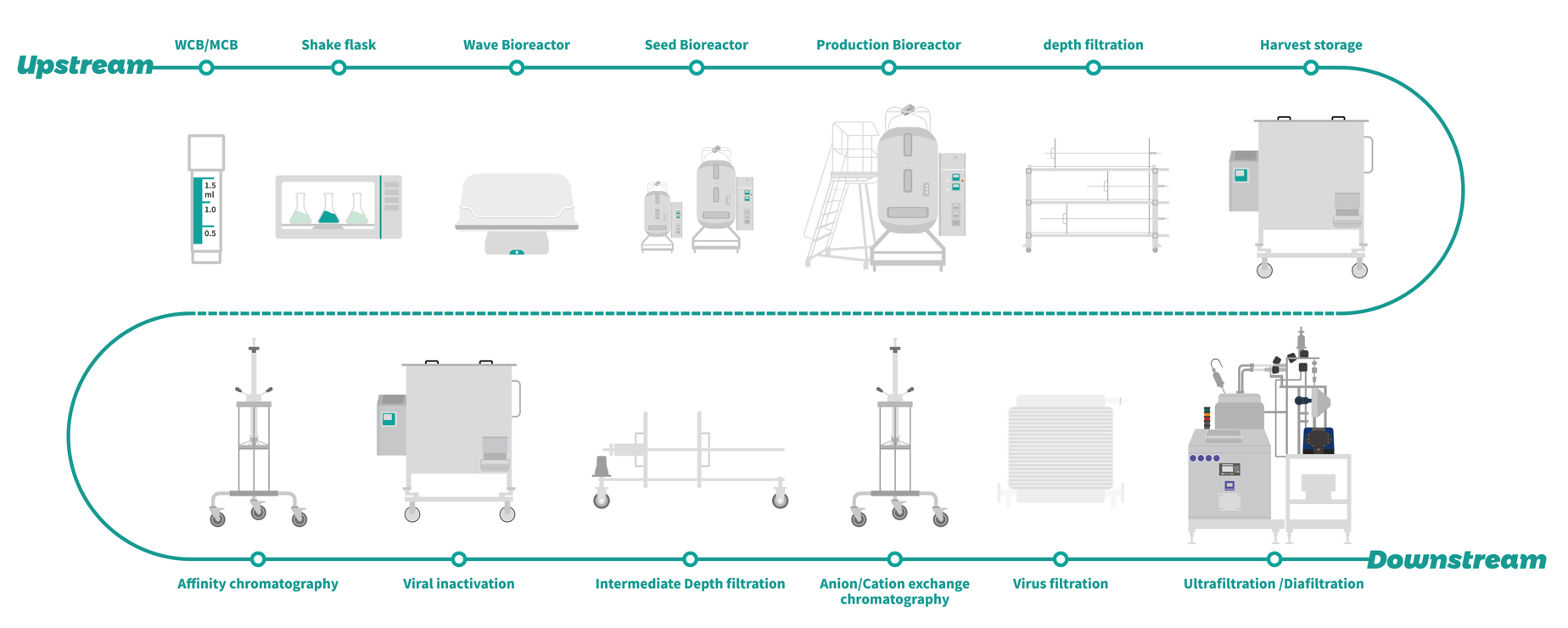
cGMP manufacturing
More than 4000 square meters of drug substance manufacturing areas.
5 separate C grade cell inoculation rooms to support cell banking and seed expansion under cGMP conditions.
Single-use platform for preparation and storage of culture medium and buffers.
Segregated upstream and downstream manufacturing areas, as well as separated downstream areas before and after virus inactivation.
Segregated Grade D support areas for upstream and downstream cleaning, sterilization, and storage.